
by Lena D. Edwards, MD, ABOIM, ABAARM, FAARM, FICT
Optimal function of all cellular and metabolic processes requires an intact circadian clock system and the predictable diurnal release of glucocortiocoids (GC). One component of the circadian clock system is the central clock, which is located within the suprachiasmatic nucleus (SCN) of the hypothalamus. Its functional integrity is affected by a number of endogenous and exogenous cues. Numerous peripheral clocks, which are located in all major organ systems, are also part of the clock system. Only through synchronization with the central clock are the peripheral clocks able to temporally orchestrate their numerous physiologic functions throughout the day. The peripheral clock located within the adrenal glands is no exception to this rule. However, unlike its counterparts, the adrenal peripheral clock serves a unique regulatory role in stabilizing the entire circadian clock system independent of central clock regulation.
It is commonly believed that stimulation of the adrenal glands and diurnal GC production is primarily reliant upon activation by the hypothalamic-pituitary-adrenal (HPA) axis. While this is true with respect to the acute stress response, regulation of the adrenal peripheral clock and diurnal GC release are predominantly under the control of the autonomic nervous system (ANS). In fact, animal studies have shown that diurnal control of GC release by the SCN-ANS-adrenal neural pathway occurs irrespective of the state of activation of the HPA axis.
The pathway through which photic stimulation, the most potent stimulator of the SCN, induces GC release is as follows:
The SCN-ANS-Adrenal Pathway
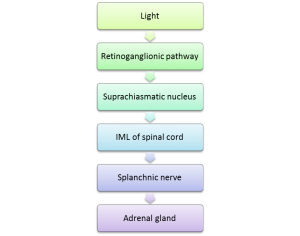
- The retinal ganglia are exposed to light
- The neural message generated is transmitted to the SCN via the retinoganglionic pathway
- From the SCN, the message travels to the intermediolateral column (IML) of the spinal cord
- From there, it travels through the neural projection from the IML to reach the splanchnic nerve
- Autonomic splanchnic nerve innervation of the adrenal gland results in GC biosynthesis and release
Photic stimuli may also directly stimulate the adrenal gland via the SCN-ANS pathway, through catecholamine- induced splanchnic nerve stimulation and through paracrine activation of the adrenal cortex by epinephrine.
There are several ways in which the adrenal peripheral clock regulates and coordinates the timing of the circadian clock system. These include modulation of GC release in response to ACTH stimulation, modification of intrinsic adrenal sensitivity to ACTH, and regulation of sympathoadrenal function and release of adrenomedullary catecholamines. Perhaps the most remarkable function of the adrenal peripheral clock is its capacity to serve as the ‘neurohumoral mediator’ between the central and peripheral clocks. In other words, the adrenal peripheral clock appears to provide stabilizing feedback from the periphery to the SCN, and coordinates reciprocal communication between the SCN and the peripheral clocks. Consequently, the entire circadian clock system is better able to resist uncoordinated shifts in response to sporadic resetting stimuli, environmental changes, and any modifications in local clock systems.
The rhythmicity of the central and peripheral clocks is also strongly affected by GC signaling. In fact, the rhythmic expression of genes in certain organs, such as the liver, is more dependent on an intact adrenal gland and GC signaling than on its own peripheral oscillator. Some of the mechanisms through which GC signaling influences the circadian clock system include:
- Modulation of the genetic expression and circadian rhythm of central peripheral clocks in parts of the brain other than the SCN
- ‘Entrainment’ or resetting of the non-central peripheral clocks
- Acts as permissive signal enabling peripheral clocks to control their own cell cycles to ensure proper signalling input to the machinery of the cell cycle
- Promotion of cyclic target gene expression independent of the molecular clocks
While substantial, the influence of the adrenal peripheral clock and GC signaling on the rhythmicity and synchronization of central and peripheral clocks can be significantly compromised if the system is subjected to improperly-timed exogenous cues. For example, if food is persistently available outside of the normal wakefulness-activity-food intake period, the rhythms of the central and peripheral clocks become desynchronized. This is partially because the central clock rhythm persists despite the presence of the superimposed dyssynchronous peripheral clock rhythms induced by ‘abnormal’ feeding times. Additionally, improperly-timed exogenous cues result in the physiologic ‘resetting’ of the adrenal peripheral clock. This results in altered adrenal responsiveness to ACTH and inappropriate, inadequate, and ill-timed GC release. Certain types of cancers, cardiovascular disease, obesity, and diabetes are only a few of the potential comorbid outcomes associated with circadian dyssynchrony.
The structural and neurohumoral components of the pathways leading to adrenal GC production are complex and diverse. During stress-induced HPA axis activation, the adrenal glands are merely end organ effectors, responding to stimulation by central hormones to produce GC. However, with respect to maintenance of SCN-ANS-adrenal axis induced circadian rhythmicity, the adrenal glands, via the adrenal peripheral clock, appears to play a much more critical and central role. A keen understanding of the differences in these regulatory pathways, the physiologic outcomes thereto, and the role the adrenal glands play is essential in properly diagnosing and treating patients with circadian dyssynchrony and abnormal salivary GC release patterns.
Click here to read the first article in this two-part series,
Timing is everything part 1: The complexity and clinical consequences of circadian dyssynchrony.
References Andrews RV, Folk GE Jr. Circadian metabolic patterns in cultured hamster adrenal glands.
Comp Biochem Physiol 1964;(11):393-409.
Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signalling.
Science. 2000; (289): 2344-2347.
Chung S, Son GH, Kim K.Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications.
Biochim Biophys Acta. 2011 May;1812(5):581-91.
Ota T, Fustin JM, Yamada H, Doi M, Okamura H. Circadian clock signals in the adrenal cortex.
Mol Cell Endocrinol. 2012 Feb 5;349(1):30-7.
Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system.
Front Neuroendocrinol. 2011 Oct;32(4):451-65.
 by Lena D. Edwards, MD, ABOIM, ABAARM, FAARM, FICT Optimal function of all cellular and metabolic processes requires an intact circadian clock system and the predictable diurnal release of glucocortiocoids (GC). One component of the circadian clock system is the central clock, which is located within the suprachiasmatic nucleus (SCN) of the hypothalamus. Its functional integrity is affected by a number of endogenous and exogenous cues. Numerous peripheral clocks, which are located in all major organ systems, are also part of the clock system. Only through synchronization with the central clock are the peripheral clocks able to temporally orchestrate their numerous physiologic functions throughout the day. The peripheral clock located within the adrenal glands is no exception to this rule. However, unlike its counterparts, the adrenal peripheral clock serves a unique regulatory role in stabilizing the entire circadian clock system independent of central clock regulation. It is commonly believed that stimulation of the adrenal glands and diurnal GC production is primarily reliant upon activation by the hypothalamic-pituitary-adrenal (HPA) axis. While this is true with respect to the acute stress response, regulation of the adrenal peripheral clock and diurnal GC release are predominantly under the control of the autonomic nervous system (ANS). In fact, animal studies have shown that diurnal control of GC release by the SCN-ANS-adrenal neural pathway occurs irrespective of the state of activation of the HPA axis. The pathway through which photic stimulation, the most potent stimulator of the SCN, induces GC release is as follows:
by Lena D. Edwards, MD, ABOIM, ABAARM, FAARM, FICT Optimal function of all cellular and metabolic processes requires an intact circadian clock system and the predictable diurnal release of glucocortiocoids (GC). One component of the circadian clock system is the central clock, which is located within the suprachiasmatic nucleus (SCN) of the hypothalamus. Its functional integrity is affected by a number of endogenous and exogenous cues. Numerous peripheral clocks, which are located in all major organ systems, are also part of the clock system. Only through synchronization with the central clock are the peripheral clocks able to temporally orchestrate their numerous physiologic functions throughout the day. The peripheral clock located within the adrenal glands is no exception to this rule. However, unlike its counterparts, the adrenal peripheral clock serves a unique regulatory role in stabilizing the entire circadian clock system independent of central clock regulation. It is commonly believed that stimulation of the adrenal glands and diurnal GC production is primarily reliant upon activation by the hypothalamic-pituitary-adrenal (HPA) axis. While this is true with respect to the acute stress response, regulation of the adrenal peripheral clock and diurnal GC release are predominantly under the control of the autonomic nervous system (ANS). In fact, animal studies have shown that diurnal control of GC release by the SCN-ANS-adrenal neural pathway occurs irrespective of the state of activation of the HPA axis. The pathway through which photic stimulation, the most potent stimulator of the SCN, induces GC release is as follows: 








.jpg.homepage-thumbnails.460x320.jpg)











SHARE