Journal Excerpt: Understanding Sleep and Sleep Disorders in Clinical Practice
Photo Cred: Shutterstock
By Natural Medicine Journal
Abstract
Sleep is an essential part of our everyday lives and can have a significant impact on health.1 It takes up one-third of the day and needs to be understood and respected for the importance it brings to our patients. There are many sleep disorders that have been recognized and are too numerous to review in this article.2 A detailed explanation of all sleep disorders can be found in the classification of sleep disorders, well-outlined by the American Academy of Sleep Medicine.3
In the first section of this article, sleep patterns and stages will be reviewed and summarized with any health information noted. To better understand the impact of sleep on health, one needs to understand basic sleep architecture. The first section will outline in detail the structure of sleep staging and significant correlations to health.
The next section will focus on the patient assessment of sleep quality and recognizing potential sleep disorders in primary care visits. The focus is on evaluating sleep disorders as a primary care practitioner. It is estimated that 50 million to 70 million people in this country alone have a chronic sleep disorder.4 This makes assessment of a patient’s sleep an extremely essential part of the primary care responsibility. Treatment will be discussed very minimally in this article.
Normal Sleep Cycle
The 2 larger sleep stages are rapid eye movement (REM) sleep and nonrapid eye movement (NREM) sleep. NREM sleep is further classified as stage 1, stage 2, and stage 3 (stage 4 is no longer used). Stages 3 and 4 sleep used to be referred to collectively as delta sleep or slow-wave sleep (SWS) and were classified as stages 3 or 4 sleep depending on the percentage of delta waves in a 30-second time interval.5
From wakefulness, an individual will begin with light sleep, which is classified as stage 1 sleep. An example of light sleep is while driving a vehicle, one experiences “drowsiness.” This is when brain waves typically found in stage 1 sleep are prevalent.6 Normal sleep involves repeating sleep stage cycles 4 to 5 times during the night (Figure 1).
REM sleep is known as dream sleep and is characterized by low-amplitude, mixed-frequency brain waves, rapid eye movements, and decreased muscle tone, also referred to as muscle atonia.7 The average adult spends around 20% to 25% of the total sleep time in this stage of sleep, which decreases slightly with age.8 There are usually 4 to 5 cycles of REM sleep, with each REM period lasting for longer periods as the night progresses (Figure 1).9 Due to the muscle atonia during REM sleep, obstructive sleep apnea (OSA) has been found to be more frequent during this stage of sleep.10 OSA is usually more prominent while people sleep supine, so theoretically OSA might be more serious during REM sleep when the patient is sleeping supine.11 This is clinically significant because if the patient is being tested for sleep apnea and body position and sleep stages are not recorded, the results may not be representative of the severity of the OSA.
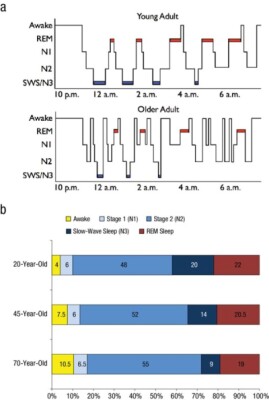
Figure 1. Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half-century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1):97-137. Used with permission from author.
REM Behavioral Disorder (RBD)
REM-related behavior disorder, which was first reported in 1986,12 is relatively uncommon in the general population (around 0.5%), but its prevalence increases up to 13% in individuals over the age of 60.13 RBD individuals act out their dreams, whereas those without the disorder have an active visual of the dream but stay in the paralyzed state during sleep. Our brain controls postural atonia, and during REM sleep, there is a loss of postural muscle tone, so humans generally will dream visually of events or activities, but their body will not act out the dreams. In RBD, an imbalance in the brainstem results in the loss of REM muscle atonia.14 In other words, the REM-sleep muscle activity in people with RBD is “tonic,” or activated instead of being inhibited.
Individuals with RBD have an increased risk of neurodegenerative diseases including dementia. In a study of 174 patients diagnosed with RBD between 1991 and 2013, a significant number of those patients (37.4%) were subsequently diagnosed with either Parkinson disease (PD), dementia with Lewy bodies, multiple-system atrophy, or mild cognitive impairment.15 RBD is common in patients with PD.16 Research at the University of Texas Southwestern Medical Center revealed that RBD was correlated with increased progression of PD (P<0.001) and could potentially be used to begin therapy.17
Dreams can be aggressive or violent in nature, an inherent concern for those who suffer from RBD, though some consider this type of behavior more of an exception than a common feature of RBD.18 Regardless, RBD is concerning and, if associated with aggressive behavior, requires intervention. This article will review the use of the drug clonazepam and the hormone melatonin for the treatment of RBD. There are no known behavior interventions that might help with this disorder; however, it is important to maintain a safe environment for those suffering from RBD. Protective padding on the floor and sleeping in a separate bedroom from their partner are all considerations to add safety.19
Clonazepam and RBD
Clonazepam, a benzodiazepine, and melatonin have traditionally been considered first-line treatment interventions for RBD. However, clinical trials have failed to support this approach.20 Clonazepam is a long-acting benzodiazepine that acts as a gamma-aminobutyric acid A (GABAA) receptor agonist. The drug also enhances the synthesis of serotonin.21 The problem with clonazepam is the side-effect profile for older adults as listed in the Beers Criteria.22 Increased sedation, fatigue drowsiness, motor impairment, and risk of falling are some of the most common side effects of this medication. In addition, the use of clonazepam is contraindicated in those with liver disease.21 In a meta-analysis, the use of benzodiazepines increases the risk of fractures (P<0.001).23 There is no evidence that clonazepam reduces the risk of developing neurodegenerative diseases or dementia.
Melatonin, RBD, and Parkinson Disease
Drugs that affect the sleep-wake cycle or circadian rhythm in humans can be classified into 3 areas. First, there are the sedative hypnotics used to treat insomnia and help initiate and maintain sleep. Benzodiazepines, such as clonazepam and lorazepam, and nonbenzodiazepines, such as zolpidem, are in this category. The second class of drugs, the stimulant medications, enhance wakefulness or alertness. Drugs in this class include armodafinil, atomoxetine, and methylphenidate. The third group, the chronobiotic drugs or substances, are meant to realign or reset the circadian rhythm.24 Melatonin is a hormone that acts as a chronobiotic agent, and this is traditionally used in the treatment of insomnia.
Melatonin has been used for years, and dosing for insomnia is usually 1to 6 mg at bedtime.25 Melatonin can be used in combination with clonazepam or as a second-line monotherapy in the treatment of RBD. In a small RBD study, the use of 3 to 9 mg of melatonin nightly significantly reduced the number of “tonic” REM activity events (P<0.01). Thirteen of the 14 subjects reported subjective improvement in RBD symptoms.26 One hypothesis of why melatonin works in RBD is that it resynchronizes the biological and circadian rhythms.27
Patients with Parkinsons disease have disturbances in biological rhythms such as heart rate, temperature, and hormonal fluctuations. It has been shown that patients with PD have elevated levels of cortisol at night and in the early morning.28 Individuals with PD develop rigidity, postural instability, and gait disturbances that can be difficult to manage. These patients also exhibit mood and sleep disorders that can lead to a decline in the quality of life. Patients with PD have an almost 6-fold increased risk of developing dementia, especially as they age.29 The cause of PD is complex and not fully delineated. PD has been linked to environmental toxin exposure, loss of dopaminergic neurons, inflammation, and oxidative damage.30
Sleep disruption and difficulty initiating and maintaining sleep are major problems in patients with PD. In a metanalysis of 7 studies looking at the benefits of melatonin for sleep disturbances in those with PD, melatonin made significant improvements in the objective and subjective quality of sleep (P=0.001).31
Melatonin possesses cytoprotective qualities and has been shown to reduce oxidative stress.32 Melatonin is synthesized primarily in the gastrointestinal tract from tryptophan, though it is also produced inside cells throughout the body and is able to cross the blood-brain barrier (BBB).33
Oral bioavailability rates vary but are relatively low, in the range of 3% to 33%.34 Doses in human trials have used as much as 100 mg daily without any signs of toxicity.35 One author suggested that if animal studies using melatonin were translated into human doses, the dose of melatonin needed to be cytoprotective would be in the range of 40 to 100 mg per day.36 The author recommended that human clinical trials are needed using these higher doses.
Editor’s Note: This article was originally published in the Natural Medicine Journal. To access the full text, click here.
References
- Ramar K, Malhotra RK, Carden KA, et al. Sleep is essential to health: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2021;17(10):2115-2119.
- Gauld C, Lopez R, Geoffroy PA, et al. A systematic analysis of ICSD-3 diagnostic criteria and proposal for further structured iteration. Sleep Med Rev. 2021;58:101439.
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders. American Academy of Sleep Medicine website. https://aasm.org/clinical-resources/international-classification-sleep-disorders/. Accessed March 31, 2024.
- National Heart, Lung and Blood Institute. What are sleep deprivation and deficiency? National Heart, Lung and Blood Institute website. https://www.nhlbi.nih.gov/health/sleep-deprivation. March 2022. Accessed March 31, 2024.
- Colten HR, Altevogt BM, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press (US); 2006.
- Purves D, Augustine GJ, Fitzpatrick D, et al, eds. Neuroscience, 2nd ed. Sunderland (MA): Sinauer Associates; 2001.
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968.
- Floyd JA, Janisse JJ, Jenuwine ES, Ager JW. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half-century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1):97-137.
- Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432-436.
- Oksenberg A, Arons E, Nasser K, Vander T, Radwan H. REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med. 2010;6(4):343-348.
- Tachibana N. [Historical overview of REM sleep behavior disorder in relation to its pathophysiology] (Japanese). Brain Nerve. 2009;61(5):558-568.
- Kang SH, Yoon IY, Lee SD, et al. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36(8):1147-1152.
- Arrigoni E, Chen MC, Fuller PM. The anatomical, cellular, and synaptic basis of motor atonia during rapid eye movement sleep. J Physiol. 2016;594(19):5391-5414.
- Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9:e89741.
- Mahmood Z, Van Patten R, Nakhla MZ, et al. REM sleep behavior disorder in Parkinson’s disease: effects on cognitive, psychiatric, and functional outcomes. J Int Neuropsychol Soc. 2020;26(9):894-905.
- Wang JJ, Dewey RB Jr. Probable REM sleep behavior disorder is a risk factor for symptom progression in parkinson disease. Front Neurol. 2021;12:651157.
- D’Agostino A, Manni R, Limosani I, et al. Challenging the myth of REM sleep behavior disorder: no evidence of heightened aggressiveness in dreams. Sleep Med. 2012;13:714-719.
- Devnani P, Fernandes R. Management of REM sleep behavior disorder: an evidence-based review. Ann Indian Acad Neurol. 2015;18:1-5.
- Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Current treatment options for REM sleep behaviour disorder. J Pers Med. 2021;11(11):1204.
- Basit H, Kahwaji CI. Clonazepam. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556010/
- American Geriatrics Society 2022 Updated AGS Beers Criteria® Update Expert Panel. 2022 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2022.
- Xing D, Ma XL, Ma JX, et al. Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporosis Int. 2014;25(1):105-120.
- Thorpy MJ, Roth T. Toward a classification of medications for sleep and circadian rhythm disorders. Nat Sci Sleep. 2013;5:143-145.
- Pierce M, Linnebur SA, Pearson SM, et al. Optimal melatonin dose in older adults: a clinical review of the literature. Sr Care Pharm. 2019;34(7):419-431.
- Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55(3):267-269.
- Videnovic A, Ju Y-eS, Arnulf I, et al. J Neurol Neurosurg Psychiatry. 2020;91:740-749.
- Hartmann A, Veldhuis JD, Deuschle M, et al. Twenty-four-hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285-289.
- Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56:730-736.
- Zafar S, Yaddanapudi SS. Parkinson Disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470193/
- Ma H, Yan J, Sun W, et al. Melatonin treatment for sleep disorders in Parkinson’s disease: a meta-analysis and systematic review. Front Aging Neurosci. 2022;14:784314.
- Lopez A, García JA, Escames G, et al. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res. 2009;46:188-198.
- Kopustinskiene DM, Bernatoniene J. Molecular mechanisms of melatonin-mediated cell protection and signaling in health and disease. Pharmaceutics. 2021;13(2):129.
- Andersen LP, Werner MU, Rosenkilde MM, et al. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol. 2016;17:8.
- Galley HF, Lowes DA, Allen L, et al. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56(4):427-438.
- Cardinali D. Are melatonin doses employed clinically adequate for melatonin-induced cytoprotection? Melatonin Res. 2019;2(2):106-132.
















