Autoimmune disease: Is a paradigm change in disease management needed?
 by David M. Brady, ND, DC, CCN, DACBN*
by David M. Brady, ND, DC, CCN, DACBN*
There is simply no doubt that the incidence of autoimmune disorders has been rising sharply over the past several decades in the Western industrialized countries, particularly the United States.1 A broad array of disorders considered immune-dysregulatory and autoimmune in nature are included in this phenomenon. The question is why has there been such a sharp rise in the incidence of these disorders?
The typical allopathic clinical approach to autoimmune disorders focuses on the management of symptoms with various anti-inflammatory medications and often the use of chemotherapeutics, and very potent immunosuppressive agents with harsh potential side-effects like leukemia and lymphoma.2 While these approaches admittedly can provide substantial symptomatic relief to the patient, they do not really get to the cause of these conditions and some research suggests that these approaches may result in a furthering of the pathological process. However, modern research into autoimmune phenomenon suggest radically different approaches may be required to reverse the above cited trends, including a strong emphasis on very early detection with predictive auto-antibodies, a focus on optimizing gastrointestinal mucosal immune function and the microbiome, eradication of infectious agent triggers with antimicrobial therapy, and even the seemingly bizarre use of parasitic agents therapeutically. Some of these concepts have a long history in naturopathic and functional models of medicine, but now are emerging as hot areas of emphasis in mainstream medical research journals and investigative communities in immunology.
Figure 1: Rising Incidence of Autoimmune Disorders
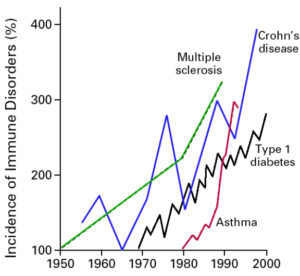
From: Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. Sep 2002;347(12):911-20.
Molecular Mimicry
The concept of molecular mimicry is really a simple one, and it is an area attracting considerable research related to the genesis of autoimmune disorders. Simply stated, environmental exposure to specific antigens (including dietary peptides and those expressed by microbes), can in genetically susceptible individuals induce cross-reactions with structurally similar amino acid motifs associated with specific host tissues. There are now multitudes of associations that have been firmly established between immune incompatibility with specific dietary-derived antigens, as well as the overgrowth of certain opportunistic and pathogenic gastrointestinal bacteria, and the presence of specific autoimmune disorders (See table 1)3-12. While some of these associations have been known for quite some time, mechanisms of causality are rapidly being established in the research. However, patients suffering from disorders like rheumatoid arthritis (RA), ankylosing spondylitis (AS), and autoimmune thyroiditis (i.e., Hashimoto’s or Grave’s disease) who visit a rheumatologist or endocrinologist do not routinely have stool analysis of their GI microbiota or food sensitivity testing performed. This is ironic, particularly in the case of opportunistic microbial overgrowth in the gut, as the conventional medical paradigm typically assumes an infectious cause, doesn’t it? Perhaps this is just another example of resistance to significant change in clinical approach within medicine, even in the face of compelling evidence to do so, as it would then require a least a passive admission that something so seemingly simple was missed for so long.
Table 1: Selected Associations of Microbial Overgrowth and Autoimmune Disorders
| Microbe Species | Disorder |
| Klebsiella | Ankylosing Spondylitis |
| Citrobacter, Klebsiella, Proteus, Porphyromonas | Rheumatoid Arthritis |
| Yersinia | Grave’s Disease & Hashimoto’s Dz. |
| S. Pyogenes | Rheumatic Fever |
| Camphylobacter | Gullian Barre Syndrome |
| Chlamydia | Multiple Sclerosis |
| E. coli, Proteus | Autoimmunity in general |
Modified from: Mayes MD. Epidemiologic studies of environmental agents and systemic autoimmune diseases. Environ Health Perspect 1999;107(suppl. 5):743-748
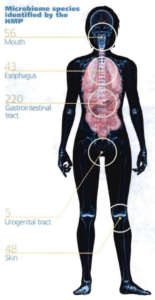
Source: Betts KS. A Study in Balance:
How Microbiomes are Changing the
Shape of Environmental Health.
Environmental Health Perspectives,
Vol. 119, No. 8, August 2011.
While all of these associations may be interesting to researchers, what does this really mean to a clinician? Some critics would argue that there is a lack of interventional data to suggest eradication of these associated organisms and/or avoidance of these dietary antigens positively affects patient outcomes. This may be true in some instances, but it has been well established, for instance, by Ebringer that successful treatment of Proteus clinically helps those with RA,7 and dietary elimination of gluten-containing grains is entirely accepted as the most viable intervention in Celiac disease. One potential issue in play is that by the time a patient is diagnosed with autoimmune disease there is often already substantial host-tissue damage. Perhaps the horse has already left the barn? However, what if potential triggers were routinely screened for and removed by health care providers, particularly in those with a family history of autoimmune disorders? The entire course of the disorder might be favorably altered, and many of these disorders might potentially never emerge clinically. In the naturopathic medicine model, there is a strong emphasis on both early detection and interventions that target the underlying pathophysiologic basis and underlying dysfunction of a disease process. Therefore, in these models the goal is to take clinical actions to reduce the potential for the disease process to progress. This also seems to intuitively make sense even in those who already have established disease; even though you may not be able to undo the damage already done, you can likely - if nothing else- slow down the train. This is particularly true since the interventions required pose little or no risk and are also relatively inexpensive; including probiotics, antimicrobial botanicals and volatile oils, mucosal-supporting nutrients and botanicals, and dietary modulation. Substantially improved molecular methods to assess the GI microbiota, utilizing PCR-DNA analysis, are also now available to clinicians at relatively low cost with rapid turn-around time.13
The Hygiene Hypothesis
The concept of the hygiene hypothesis is also one that is quite simple, with the complexity being in the details. The thought that we have induced dysregulation into our immune system’s by virtue of living in too clean of an environment and the over eradication of infection is not new, but it has gained favor with researchers who have begun to work out exactly why this may be the case. Some of these concepts were elegantly addressed by Weiss in an editorial in the New England Journal of Medicine entitled Eat Dirt-The Hygiene Hypothesis and Allergic Disease.14 While there is no doubt that modern public health measures, such as adequate sewage systems, water treatment, the use of antibiotic agents, and various other aspects of modern hygiene have lessened deadly infectious outbreaks and have prevented unnecessary deaths. However, as with most things, there is a yin and yang. This “new clean world” has likely resulted in a lack of adequate sampling of our environment, including a lack of exposure to all of the microbes that we share our planet with, particularly while we are young and our immune systems are developing the delicate balance between adequate defense and tolerance.15
*University of Bridgeport, Division of Health Sciences, Bridgeport, Connecticut, U.S.
The Role of Parasites
As reported by David Gutierrez in NaturalNews, researchers in a study conducted at the University of Nottingham, point out that humans and gastrointestinal parasites might have co-evolved in a way that the parasites actually help regulate the human immune system to prevent allergies.16 They believe that over the course of millions of years, gastrointestinal parasites have evolved the ability to suppress the human immune system as a survival mechanism. Because parasitic infestation has been so common throughout human evolutionary history, the human immune system has in turn evolved to compensate for this effect. This means that if the parasites are removed, the immune system may actually function too strongly, resulting in maladaptive immune responses such as asthma, allergies, and eczema.
With issues such as the hygiene hypothesis, and the role of parasites in immune function in mind, gastroenterologist and researcher Dr. Joel Weinstock, originally at the University of Iowa, and now Tufts University, has performed novel work with subjects with inflammatory bowel disease (IBD).17 IBD was unheard of before the 20th century. Beginning of 20th century incidence is thought to be about 1:10,000 and is now 1:250. Similar data exists with the incidences of asthma, hay fever, DM, MS, etc. Weinstock conducted various studies of IBD patients and treated them with the therapeutic parasite Trichuris suis, a porcine whipworm, which was an ideal choice as it only remains viable in the human GI tract for a short time and must be continually administered. The organism, when introduced into patients with IBD; 1) induced changes in regulatory T cell function; 2) blocked T cell proliferation; 3) altered cytokine production and expression of innate immunity; 4) altered the intestinal flora; and 5) generally produced a lessening of symptoms and severity of disease. Pharmaceutical agents are now being developed along these lines to treat IBD.
Intestinal Hyper-permeability (aka: “Leaky Gut Syndrome”)
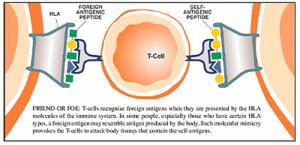
Leaky gut syndrome for much of the past twenty years seemed something that just naturopathic and functional medicine doctors talked about. Not any longer! Prestigious researchers such as Alessio Fasano at the University of Maryland, have been researching the role of intestinal permeability in the pathogenesis of autoimmune disorders and bringing this concept full-speed to the conventional medical research community through his publications in top-tier immunology and gastroenterology journals.18 In a 2009 article in Scientific American he eloquently brought the topic to the lay audience with his article Surprises from Celiac Disease, where he described that his theory that leaky gut contributes to Celiac disease and autoimmunity was initially greeted with skepticism by his colleagues.19 Fasano has proposed that in order for autoimmune disease to manifest there must be three factors present, and he equates these to a triangle, or three-legged stool, where if any are not present the disease cannot exist. These three factors include; 1) an environmental trigger (i.e., antigen), 2) genetic susceptibility (i.e., an HLA pattern that is particularly efficient at presenting the antigen to the immune cells, such as the presence of the HLA-DQ2 and HLA-DQ2 pattern in Celiac disease), and 3) intestinal hyper-permeability (i.e., “leaky gut syndrome”). He goes on to opine that by far the easiest of these three factors to alter clinically is intestinal permeability. Much of his work, and others, involves the study, and future therapeutic manipulation, of a protein which alters intestinal permeability by the name of zonulin.20
Fasano summarizes the role of intestinal mucosal health and hyper-permeability in autoimmunity best in a 2005 paper when he states, “Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to nonself-antigens. When the finely tuned trafficking of macromolecules is dysregulated in genetically susceptible individuals, both intestinal and extraintestinal autoimmune disorders can occur.”18
 Naturopathic physicians, and other nutritionally-minded providers, have been addressing the issue of leaky gut for a long time with effective natural agents, including; L-glutamine, N-acetyl-glucosamine, anti-inflammatory botanicals and bioflavonoids, mucilaginous herbs, zinc-carnosine, omega-3-fatty acids and more. However, one popular nutrient that is used frequently as an immune modulator in autoimmune conditions is vitamin D. However, most clinicians are not aware of the role vitamin D plays directly in intestinal permeability. According to Kong et al in their 2008 paper entitled Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Gastrointestinal Barrier, “In vitro experiments demonstrate that the VDR mediates the activity of 1,25(OH)2D3 that induces junction protein expression and strengthens the tight junction complex. These data are consistent with, and explain at least in part, the observation reported in the literature that vitamin D deficiency is linked to increased incidence of IBD in human population.”21
Naturopathic physicians, and other nutritionally-minded providers, have been addressing the issue of leaky gut for a long time with effective natural agents, including; L-glutamine, N-acetyl-glucosamine, anti-inflammatory botanicals and bioflavonoids, mucilaginous herbs, zinc-carnosine, omega-3-fatty acids and more. However, one popular nutrient that is used frequently as an immune modulator in autoimmune conditions is vitamin D. However, most clinicians are not aware of the role vitamin D plays directly in intestinal permeability. According to Kong et al in their 2008 paper entitled Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Gastrointestinal Barrier, “In vitro experiments demonstrate that the VDR mediates the activity of 1,25(OH)2D3 that induces junction protein expression and strengthens the tight junction complex. These data are consistent with, and explain at least in part, the observation reported in the literature that vitamin D deficiency is linked to increased incidence of IBD in human population.”21
Another possible role for vitamin D in the treatment of autoimmune disease, including MS, involves antimicrobial action. In addition to the previously cited findings by Harkiolaki et al regarding molecular mimicry induced by various GI bacteria in MS,9 researchers like Dr. Charles Stratton at Vanderbilt University have made clear associations between MS and Chlamydia pneumoniae,22 and others, including Dr. Donald Gilden, have implicated various viral triggers in MS.23 It has also been shown that the human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25 dihydroxyvitamin D3.24 Meaning, as vitamin D levels rise, so does the production of this endogenous antimicrobial peptide in the body, and this may account for some of the clinical benefit observed with vitamin D therapy in MS and other autoimmune disorders.
Predictive Autoantibody Testing (A True Application Preventive Medicine?)
In a 2007 Scientific American article entitled New Predictors of Disease, Abner Louis Notkins stated “Molecules called predictive autoantibodies appear in blood years before people show symptoms of various disorders. Tests that detect these molecules could warn of the need to take preventive action.”25 While some of these tests have been used for many years in a very selective manner, often simply to confirm the presence of a disease strongly suspected by clinical presentation and examination. However, the development and availability of low-cost autoantibody arrays has ushered in the possibility to use autoantibody testing in a much more proactive screening strategy to predict the future emergence of autoimmune disorders so that preventive action can be initiated early to short-circuit the disease process.26 Table 2 outlines some of the available predictive autoantibody tests, their positive predictive value (PPV), and the years before clinical diagnosis that they generally appear in the blood of subjects with specific disorders.27-29 As inexpensive tests for predictive autoantibodies continue to be developed, they could become part of a routine check-up, particularly by preventive physicians such as naturopathic and functional medicine physicians.30
Table 2: Selected Predictive Autoantibody Tests
| Disease/Disorder | Autoantibody Tests | Positive Predictive Value | Years Prior to Clinical Diagnosis |
| Addison’s Disease | *Adrenal cortex antibodies | 70 | 10 |
| Celiac Disease | *Anti-tissue transglutaminase *Anti-endomysial antibodies *HLA-DQ2 or DQ8 antigens | 50-60% 50-60% 100% | 7 |
| Hashimoto’s Thyroiditis | *Anti-thyroid peroxidase antibodies (postpartum) | 92% | 7-10 |
| Primary Biliary Cirrhosis | *Anti-mitochondrial antibodies | 95% | 26 |
| Rheumatoid Arthritis | *Rheumatoid factor *Anti-cyclic citrullinated peptide | 62-88% 97% | 14 |
| Scleroderma | *Anti-centromere antibodies *Anti-topoisomerase I antibodies | 100% | 11 |
| Sjogren’s Syndrome | *Anti-Ro and La antibodies | 73% | 5 |
| SLE | *RNP, Sm, dsDNA, Ro, La, and cardioliptin antibodies | 94-100% | 7-10 |
| Type I Diabetes | *Pancreatic islet cell *Insulin *65 kD glutamic acid decarboxylase *Tyrosine phosphatase-like protein | 43% 55% 42% 29% | 14 |
O’Bryan T, American College for Advancement in Medicine annual symposium presentation 2009.
Shoenfeld Y, Blank M, Abu-Shakra M, et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune disease. IMAJ, 2008;10:13-19.
Lindberg B, Iverson SA, et al. Islet autoantibodies in cord blood in children who develop Type I (insulin-dependent) diabetes mellitus before 15 years of age. Diabeteologia, 1999;42:181-187.
Summary
It is hoped that this article will help the physician to develop a comprehensive conceptual framework from which to view autoimmune disease and to institute a new proactive clinical model from which to evaluate patients. Physicians should look for immune dysregulatory conditions with a strong emphasis on: 1) very early detection with predictive auto-antibodies; 2) a focus on optimizing gastrointestinal mucosal immune function and the microbiome; 3) the eradication of infectious triggers with antimicrobial therapy; 4) the detection and elimination of food sensitivities; and 5) the promotion of an anti-inflammatory lifestyle.
About the Author
Dr. David M. Brady is a Connecticut and Vermont licensed naturopathic medical physician, a doctor of chiropractic, and certified clinical nutritionist. He is the vice president of the Division of Health Sciences, director of the Human Nutrition Institute, and associate professor of clinical sciences at the University of Bridgeport. He is also the chief medical officer for Designs for Health, Inc. and Diagnostic Solutions Labs, LLC, and practices at Whole Body Medicine in Fairfield, CT, specializing in functional and nutritional medicine.
References:
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. Sep 2002;347(12):911-920.
- Inaba M, Ushijim S, Hirata N, et al. Methotrexate-related lyphomatoid granulomatosis in a patient with rheumatoid arthritis. Nihon Kokyuki Gakkai Zasshi (Article in Japanese). Aug 2011;49(8):597-601.
- Mayes MD. Epidemiologic studies of environmental agents and systemic autoimmune diseases. Environ Health Perspect 1999;107(suppl. 5):743-748
- Pishak OV. Bukovian State Medical Academy, Public Health Ministry of Ukraine. Mikrobiol Z. Sep-Oct 1999;61(5):41-47.
- Tiwana H, Wilson C, Walmsley RS, et al. Antibody responses to gut bacteria in ankylosing spondylitis, rheumatoid arthritis, Crohn’s disease and ulcerative colitis. Rheumatol Int. 1997;17:11-16.
- Ebringer A, Rahid T. Rheumatoid arthritis is an autoimmune disease triggered by Proteus urinary tract infection. Clin Dev Immunol. Mar 2006;13(1):41-48.
- Ebringer A, Rahid T, Wilson C. Rheumatoid arthritis: proposal for the use of anti-microbial therapy in early cases. Scand J Rheumatol. 2003;32:2-11.
- Liao F, Li Z, Wang Y, et al. Porphyromonas gingivalis may play an important role in the pathogenesis of periodontitis-associated rheumatoid arthritis. Med Hypotheses. Feb 2009;72;732-735.
- Harkiolaki M, Holmes SL, Svendsen P, et al. T-cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity. 20 Mar, 2009;30:348-357.
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 29 May 2008;453(7195):620-625.
- Petru G, Stunzner D, Lind P, et al. Antibodies to Yersinia enterocolitica in immunogenic thyroid diseases. Acta Med Austriaca (Article in German). 1987;14(1):11-14.
- Anasaldi N, Palmas T, Corrias A, et al. Autoimmune thyroid disease and celiac disease in children. J Pediatr Gastroenterol Nutr. Jul 2003;37(1):63-66.
- Brady D. Novel Options in GI Diagnostics: DNA Detection of Gut Microbiota. Complementary Med. Jul-Aug 2008:28-31.
- Weiss ST. Eat dirt-the hygiene hypothesis and allergic disease (editorial). N Engl J Med. 19 Sep 2002;347(12):930-931.
- von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. Dec 2010;10(12):861-868.
- Gutierrez D. Parasites in your gut actually help protect you from allergies. NaturalNews. Available at: http://www.naturalnews.com/028141_parasites_allergies.html. Accessed on Nov 03, 2011.
- Summers RW, Elliott DE, Weinstock JV, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. Sep 2003;98(9):2034-2041.
- Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. Sept 2005;2(9):416-422.
- Fasano A. Surprises from celiac disease. Sci Am. Aug 2009:301(2):54-61.
- Sapone A, de Magistris L, Pietzak M. Zonulin upregulation is associated with increased gut permeability in subjects with type I diabetes and their relatives. Diabetes. May 2006;55(5):1443-1449.
- Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastro Liver Physiol. 2008;294:G208-G216.
- Yao SY, Stratton CW, Mitchell WM. CSF oligoclonal bands in MS include antibodies against Chlamydophilia antigens. Neurology. 2001;56:1168-1176.
- Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195-202.
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-hihydroxyvitamin D3. Future Microbiol. 2009;4(9):1151-1165.
- Notkins AL. New predictors of disease. Sci Am. 2007;296(3):72-79.
- Leslie D, Lipsky P, Notkins AL. Autoantibodies as predictors of disease. J Clin Invest. 2001;108:1417-1422.
- O’Bryan T, American College for Advancement in Medicine annual symposium presentation 2009.
- Shoenfeld Y, Blank M, Abu-Shakra M, et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune disease. IMAJ, 2008;10:13-19.
- Lindberg B, Iverson SA, et al. Islet autoantibodies in cord blood in children who develop Type I (insulin-dependent) diabetes mellitus before 15 years of age. Diabeteologia, 1999;42:181-187.
- Vojdani A. Antibodies as predictors of complex autoimmune diseases and cancer. Int J Immunopathol Pharmacol. Jul-Sep 2008;21(3):553-566.
Editor's note: Brady will be presenting at the Integrative Healthcare Symposium Annual Conference, which will be held February 22-24, 2018 in New York City. IntegrativePractitioner.com is the official media for the Integrative Healthcare Symposium. This article is part of the our IHS Preview Series, a collection of articles, interviews, and podcasts where we offer a first look at some of the exciting topics offered at this year's event. Click here for more information.



















SHARE